Background:
Overexpression of anti-apoptotic proteins such as MCL-1 has been implicated in resistance to the BCL2 inhibitor venetoclax in relapsed/refractory AML (R/R-AML). Therapeutic agents that reduce resistance to venetoclax as well as exhibiting intrinsic anti-tumor activity may synergize with venetoclax to increase response rates in this difficult to treat patient population. One approach to enhance the potency of venetoclax is through combination with targeted radiotherapy. Studies have shown that radiation-induced DNA damage reduces the level of MCL1 in tumor cells. The monoclonal antibody radioconjugate lintuzumab-225Ac is a highly cytotoxic alpha-radiation emitter that selectively targets CD33, a cell surface antigen expressed on the majority of AML cells but not on non-hematopoietic tissues. Prior clinical studies with lintuzumab-225Ac have demonstrated potent single agent anti-leukemic activity (ASH 2017, 2018). The short range, high-energy alpha-particle emissions from lintuzumab-225Ac elicit single and double-strand DNA breaks in targeted tumor cells. In venetoclax-resistant cell lines in vitro, lintuzumab-225Ac promotes MCL1 degradation resulting in increased cell sensitivity to venetoclax. In vivo mouse xenograft models with venetoclax-resistant AML tumor lines demonstrated enhanced tumor regression and increased survival in mice receiving both venetoclax and lintuzumab-225Ac. This study is the first study to combine radioimmunotherapy with venetoclax in patients with R/R-AML.
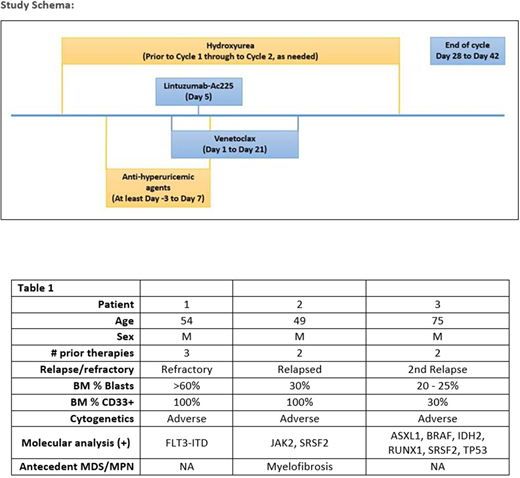
Study Design:
The Phase I portion of the study will use a 3+3 dose-escalation design to determine the maximum tolerated dose (MTD) of lintuzumab-225Ac when given in combination with venetoclax. The planned dose levels for lintuzumab-225Ac are 0.5, 1.0, and 1.5 µCi/kg. As an exploratory endpoint, BH3 profiling will be performed on patient marrow samples to assess for MCL1 activity.
Eligible patients include R/R-AML patients aged 18 years and older with adequate organ function, ECOG Performance Status 0-2, and more than 25% of leukemic blasts CD33 positive by flow cytometry. Patients with antecedent MDS, MPNs, or therapy-related AML are eligible. Other than the cycle 1 dose ramp up to reduce the risk of tumor lysis syndrome, patients receive venetoclax at 400 mg/day PO on Days 1 to 21 of each cycle with dose modification for the use of CYP3A inhibitors. The Lintuzumab-225Ac is administered as a single dose on Day 1 of each cycle (see Study Schema).
Results:
Three R/R AML patients with a median age of 54 years (range 49-75) have been enrolled to date (see Table 1). The enrolled patients had a median of 2 therapies (2-3) and a median bone marrow blast percentage of 30% (range 20 - >60). All 3 patients had poor risk with adverse cytogenetics, and each patient has an additional high-risk marker (FLT3-ITD+, antecedent JAK2+ myelofibrosis, or TP53 mutation).
There have been no lintuzumab-225Ac related dose limiting toxicities (DLT) or non-hematologic Grade 3 or greater related AEs.
Patient 1 obtained a partial response with a decrease in BM leukemic blasts from >60% to 30-40% after cycle 1. Efficacy in the other 2 patients at this dose level is too early to evaluate.
Conclusion:
Combining lintuzumab Ac225 with venetoclax in patients with R/R-AML has an acceptable initial clinical safety profile at the level of 0.5 µCi/kg of lintuzumab-225Ac. Results in the first lintuzumab-225Ac dose cohort are encouraging, and the trial will continue to enroll to evaluate the hypothesis that there will be clinical synergy consistent with pre-clinical studies.
Hegazi:Tempus: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy. Harpel:Actinium Pharmaceuticals, Inc: Current Employment, Current equity holder in publicly-traded company. Miao:Actinium Pharmaceuticals, Inc: Current Employment, Current equity holder in publicly-traded company. Ludwig:Actinium Pharmaceuticals, Inc: Current Employment, Current equity holder in publicly-traded company. Berger:Actinium Pharmaceuticals Inc.: Current Employment, Current equity holder in publicly-traded company. Schiller:Abbvie: Research Funding; Kaiser Permanente: Consultancy; Geron: Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Kite Pharma: Research Funding; Karyopharm: Research Funding; DeltaFly: Research Funding; Gamida: Research Funding; Bristol-Myers Squibb: Current equity holder in publicly-traded company, Research Funding; FujiFilm: Research Funding; Forma: Research Funding; Jazz Pharmaceuticals: Research Funding; MedImmune: Research Funding; Onconova: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; Regimmune: Research Funding; Samus: Research Funding; Sangamo: Research Funding; Tolero: Research Funding; Trovagene: Research Funding; Mateon: Research Funding; Amgen: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Agios: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Ariad: Research Funding; Stemline: Speakers Bureau; Actinium: Research Funding; Incyte: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Ono Pharma: Consultancy; Celgene: Research Funding, Speakers Bureau; Sanofi: Speakers Bureau; Gilead: Speakers Bureau; Astellas Pharma: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; Cyclacel: Research Funding; Constellation: Research Funding; Celator: Research Funding; Deciphera: Research Funding; Genentech-Roche: Research Funding.
Venetoclax use in relapsed-refractory AML.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal